Question 1.
Match the pairs.
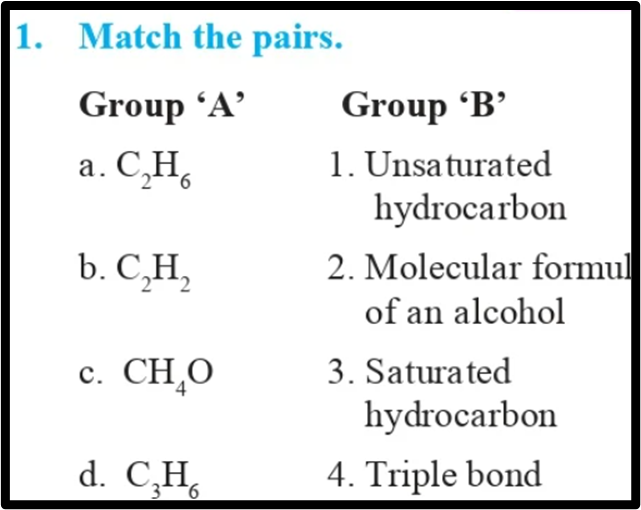
Answer
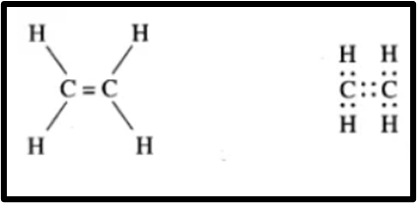
Question 2.Draw an electron dot structure of the following molecules. (Without showing the circles)
(a)Methane

(b)Ethene
(c ) Methanol

(d) Water
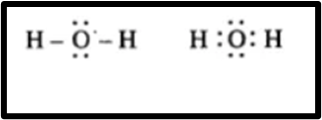
Question 3. Draw all possible structural formulae of compounds from compounds from their molecular formula given below.
Answer. (a) C3H8 Propane
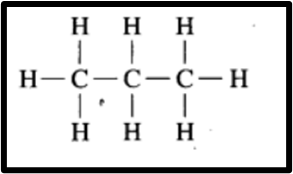
(b) C4H10 Butane

(c) C3H4 Propyne

Question 4. Explain the following terms with example.
a. Structural isomerism.
Structural isomerism occurs when compounds with different structural formulas share the same molecular formula. For instance, butane is depicted by two distinct compounds due to their differing structural formulas. One compound forms a linear chain, while the other forms a branched chain. Despite this distinction, both compounds share the molecular formula C4H10.

Two structural Isomers of Butane (C4H10)
b. Covalent bond.
A covalent bond occurs when two atoms share pairs of electrons, also known as a molecular bond. The interaction between atoms sharing an electron pair is referred to as covalent bonding.
Answer. Hydrogen Molecule Formation: Hydrogen has an atomic number of 1, with one electron in its K shell. To complete the K shell and achieve the configuration of helium (He), hydrogen needs one additional electron. To fulfill this need, two hydrogen atoms share their electrons, resulting in the formation of an H2 molecule through the creation of a single covalent bond, or a single bond, by sharing two electrons.

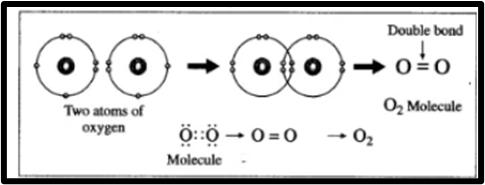
2. Formation of oxygen molecule:
1. Oxygen has an atomic number of 8, with an electronic configuration of (2, 6) and 6 electrons in its outermost shell. 2. To achieve the configuration of neon (Ne), the L shell of oxygen needs 2 additional electrons. 3. In the formation of an oxygen molecule, each oxygen atom shares its valence electron with another oxygen atom, resulting in two shared pairs of electrons. 4. Consequently, a double covalent bond is formed between two oxygen atoms through the sharing of two electron pairs.
c. Hetero atom in a carbon compound.
Answer. In a carbon compound, a heteroatom is an atom other than carbon or hydrogen that replaces carbon and hydrogen atoms. Common heteroatoms include nitrogen, oxygen, sulfur, phosphorus, chlorine, bromine, and iodine.

d. Functional group.
The presence of hetero atoms or groups containing hetero atoms gives compounds distinct chemical properties, regardless of the carbon chain’s length and nature in the compound. These hetero atoms or groups are known as functional groups.
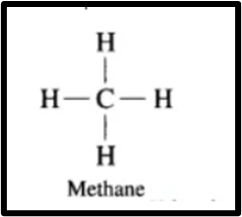
e. Alkane. The carbon atom in hydrocarbons forms single bonds to satisfy its four valencies, making these compounds known as alkanes.
Example: Methane consists of a carbon atom bonded to four hydrogen atoms through four single covalent bonds.
f. Unsaturated hydrocarbons.
The term “unsaturated” describes a compound that has double or triple bonds, meaning not every carbon is bonded to a different atom. Ethene (C2H4) is an example of an unsaturated hydrocarbon, along with other compounds like benzene (C6H6) and acetic acid (C2H4O2).
g. Homopolymer.
A homopolymer consists of a single type of monomer unit. Examples include polyethylene (with ethylene as the monomer unit) and PVC (with vinyl chloride as the monomer unit).
h. Monomer : The monomer is the basic unit that repeats consistently to create a polymer. The monomers include glucose, vinyl chloride, amino acids, and ethylene.
I. Reduction: When oxygen is taken away from a compound or hydrogen is added to a compound in a chemical reaction, it is known as reduction.
j. Oxidant.: Oxidants are substances that oxidize or remove electrons from other substances in a redox reaction. They can also be referred to as oxidizers or oxidizing agents. If the oxidant contains oxygen, it may be known as an oxygenation reagent or oxygen-atom transfer (OT) agent. Some examples of oxidants are hydrogen peroxide, ozone, nitric acid, and sulfuric acid.
Question 5. Write the IUPAC names of the following structural formulae.
a. CH3 – CH2 – CH2 – CH3
Answer: The longest chain contains 4 carbon atoms. Parent alkane: Butane IUPAC name: n-Butane
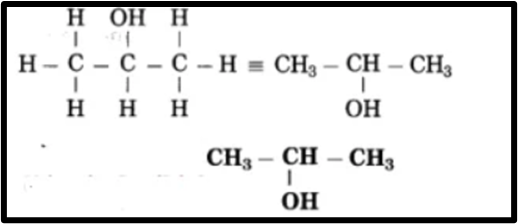
b. CH3 – CHOH – CH3Answer.Longest chain contains 3 carbon atoms
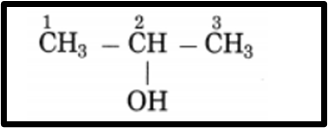
The parent alkane is propane with a functional group of -OH (ol) attached to the carbon atom numbered as C2. The ending of the parent name is changed from ‘e’ to ‘ol’ to indicate the presence of the -OH group. This compound is named as Propan-2-ol in IUPAC nomenclature.
c. CH3 – CH2 – COOH
The longest chain has 3 carbon atoms. The parent alkane is Propane. The functional group is -COOH (-oic acid). If the carbon chain includes a -COOH group, the parent name’s ending changes, so ‘e’ in propane becomes ‘oic acid’. Therefore, the parent suffix is Propanoic acid and the IUPAC name is Propanoic acid.
d. CH3 – CH2 – NH2Carbon atom count: 2 Parent alkane: Ethane Functional group: -NH2 (amine) When a -NH2 group is present in the carbon chain of the compound, the parent name ending is altered, changing ‘e’ in ethane to ‘amine’. Parent suffix: Ethanamine IUPAC name: Ethanamine.
e. CH3 – CHO
Answer: Carbon Atom Count: 2 – Base Alkane: Ethane – Functional Group: -CHO (al) – When the carbon chain includes a -CHO group, modify the ending of the base name, such as replacing the ‘e’ in ethane with ‘al’. – Base Suffix: Ethanal – IUPAC Designation: Ethanal
f. CH3 – CO – CH2 – CH3
Longest carbon chain: 4 carbon atoms Main alkane: Butane Functional group: -CO- (singular) Assign the number:
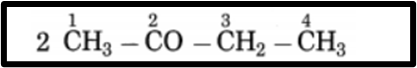
The carbon atom numbering begins from the carbon atom closest to the functional group within the longest chain. If the compound’s carbon chain includes a (-CO-) group, modify the suffix of the main name, for instance, substitute the ‘e’ in butane with ‘one’. parent suffix: Butan-2-one IUPAC name: Butan-2-one
Question 6.
Identify the type of the following reaction of carbon compounds.
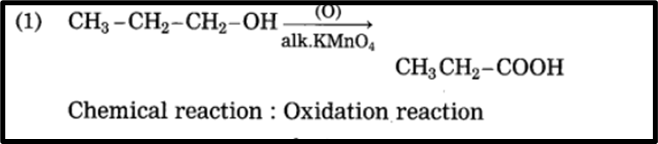
1. CH3 – CH2 – CH2 – OH + (O) → CH3 – CH2 – COOH
2. CH3 – CH2 – CH3 + O2 → 3CO2 + 4H2O

3. CH3 – CH = CH – CH3 + Br2 → CH3 – CHBr – CHBr – CH3

4. CH3 – CH3 + Cl2 → CH3 – CH2 – Cl + HCl

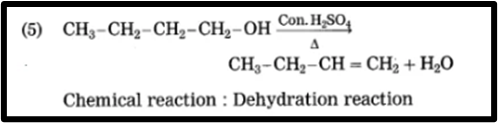
5. CH3 – CH2 – CH2 – CH2 – OH → CH3 – CH2 – CH = CH2 + H2O
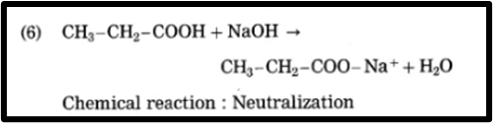
6.CH3 – CH2 – COOH + NaOH → CH3 – CH2 – COONa+ + H2O
7. CH3 – COOH + CH3 – OH → CH3 – COO – CH3 + H2O

Question7.Write the structural formulae for the following IUPAC names:
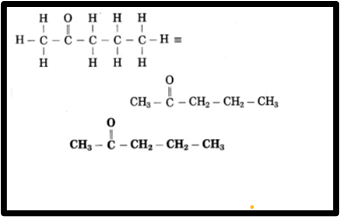
a. Pent-2-one
(1) Pent-2-one is a compound where the prefix “pent” indicates a chain of five carbon atoms and the suffix
“one” signifies the presence of a specific functional group.
The ketone group is located at carbon 2 (C2) in the molecule
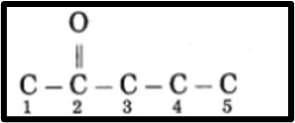
(3) Now, fulfil the valences of each carbon atom.
b. 2-Chlorobutane
(1) In 2-chlorobutane, butane is the parent alkane representing 4 carbon atoms, and the carbon atoms in the chain are numbered as 1, 2, 3, and so on.
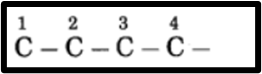
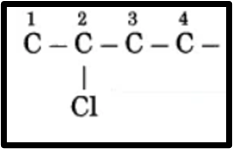
(2) The prefix for chloro is Halo. The assigned number for the chloro prefix is 2. Display the chloro atom at C2.

(3) Now, fulfill the valences of each carbon atom.
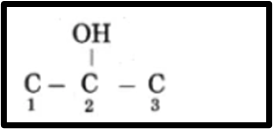
© Propan-2-ol
(1)Propan represents a chain of three carbon atoms, which are numbered as 1, 2, and 3.

(2)The suffix “-ol” denotes the presence of a hydroxyl group (-OH). The hydroxyl group is located at position 2 on the molecule, represented as -OH on C2.
(3) Now, fulfill the valences of each carbon atom.
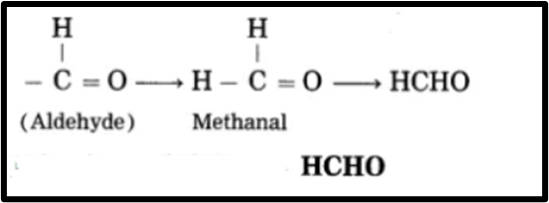
d. Methanal
1. Meth represents one carbon atom and designates the carbon as ‘1’ in the functional group -CHO.
2. ‘-al’ denotes the functional group (-CHO) aldehyde.
3. Fulfill the carbon’s valencies in -CHO now.
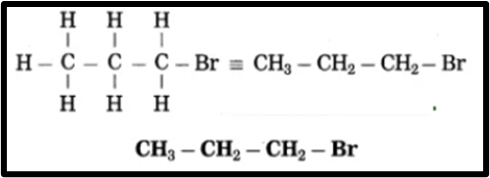
f. 1-Bromopropane.
(1) In 1-bromopropane, the parent alkane is propane, which signifies three carbon atoms. The carbon atoms in the chain are numbered as 1, 2, 3, and so on.
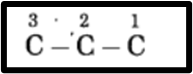
(2) The prefix “Bromo” indicates the bromine atom is at carbon 1 in a molecule, with the prefix number being 1.
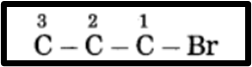
(3) Now, fulfill the valences of each carbon atom
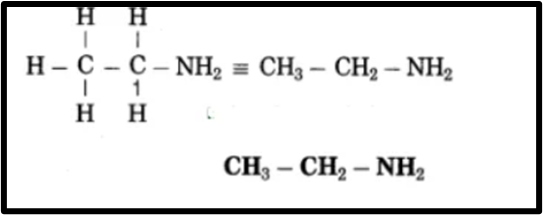
g. Ethanamine
(1) Eth represents a chain of 2 carbon atoms, with ethane being the parent alkane. – C – C –
(2) The term ‘amine’ refers to the (- NH2) amino group. Display the amino (-NH2) at a carbon atom. – C – C – NH2
(3) Now, fulfill the valences of each carbon atom
h. Butanone.
(1.) “But” represents 4 carbon atoms in a chain, with the parent alkane being butane. These carbon atoms are numbered consecutively as 1, 2, 3, and so on.
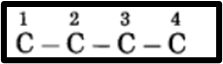
(2.) “One” denotes a functional group.

The ketone group is located at carbon 2 and is represented as C2.
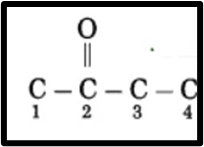
(3) Now, fulfill the valences of each carbon atom
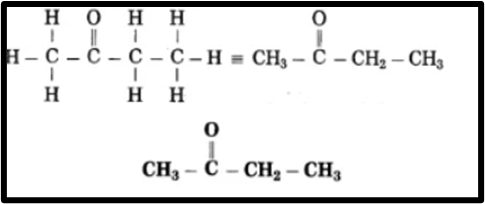
Question 8.
a. What causes the existance of very large number of carbon compound?
Answer.1. Carbon can form strong covalent bonds with other carbon atoms, leading to the creation of large molecules known as catenation power. Carbon compounds can consist of either open chains or closed chains of carbon atoms, with open chains being straight or branched, and closed chains forming ring structures. The robust and stable covalent bond between two carbon atoms contributes to carbon’s catenation power.
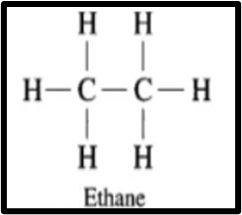
2. Two carbon atoms can bond together with one, two, or three covalent bonds, referred to as single, double, and triple covalent bonds, respectively. With the ability to form both multiple and single bonds, carbon compounds exhibit a wide range of chemical diversity. For instance, ethane (CH3 – CH3), ethene (CH2 = CH2), and ethyne (CH = CH) are examples of compounds containing two carbon atoms.
3. Carbon, being tetravalent, can form bonds with up to four other atoms, including carbon and other elements, resulting in the formation of numerous compounds. These compounds display varied properties based on the atoms bonded to carbon. For instance, by bonding one carbon atom with hydrogen and chlorine, five different compounds are generated: CH4, CH3Cl, CH2Cl2, CHCl3, CCl4. Carbon atoms also create covalent bonds with elements such as O, N, S, halogens, and P, yielding a diverse array of carbon compounds.
4. Isomerism is another defining characteristic of carbon compounds, contributing significantly to the vast number of carbon compounds in existence.
b. Saturated hydrocarbons are classified into three types. Write these names giving one example each.
Answer. Hydrocarbons with all four valencies of a carbon atom filled by single bonds are referred to as saturated hydrocarbons. Methane consists of a single carbon atom to which four hydrogen atoms are bonded through four covalent bonds.
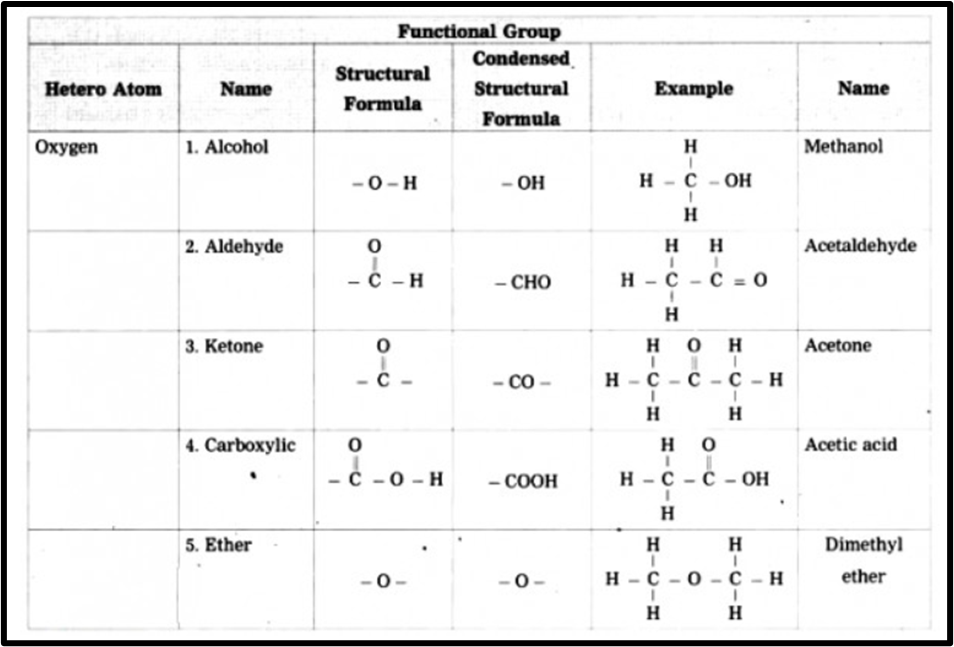
c. Give any four functional groups containing oxygen as the heteroatom in it. write name and structural formula of one example each.
Answer. Functional group containing oxygen
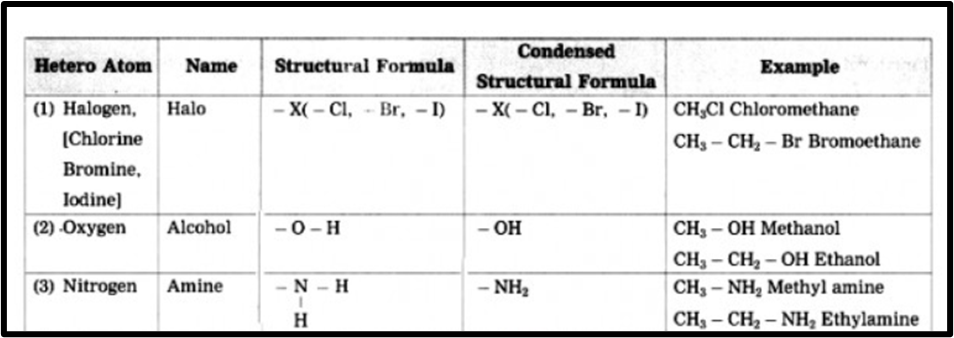
d. Give names of three functional groups containing three different heteroatoms. Write name and structural formula of one example each.
Answer. Three functional group containing
e. Give names of three natural polymers. write the place of their occurance and names of monomers from which they are formed.
Answer
- Polysaccharide is a natural polymer found in starch and carbohydrates, created from glucose monomers.
- Protein, a natural polymer made up of alpha amino acids, is present in muscles, hair, enzymes, skin, and eggs.
- Rubber, another natural polymer, can be found in the latex of rubber trees and is composed of isoprene monomers.
f. What is meant by vinegar and gasohol? What are their uses?
Answer :1. Vinegar is a solution containing 5 to 8% acetic acid in water. It serves as a preservative in pickles, for cooking meat, and as a salad dressing.
2. Gasohol is a fuel made by mixing petrol with 10% anhydrous ethanol to enhance its efficiency. This fuel is commonly used in cars and other vehicles.
g. what is a catalyst ? write any one reaction which is brought about by use of catalyst?
Answer: A catalyst is a substance that alters the reaction rate without interfering with it. When vegetable oil (an unsaturated compound) reacts with hydrogen in the presence of a nickel catalyst, it transforms into vanaspati ghee (a saturated compound) through an addition reaction.