Question 1.
Fill in the blanks and rewrite the sentences:
a. The amount of water vapour in air is determined in terms of its………..
Answer: The amount of water vapour in air is determined in terms of its absolute humidity.
b. If objects of equal masses are given equal heat, their final temperature will be different. This is due to difference in their………….
Answer:
If objects of equal masses are given equal heat, their final temperature will be different. This is due to difference in their specific heat capacities
Question 2.
Observe the following graph. Considering the change in volume of water as its temperature is raised from 0 °C, discuss the difference in the behaviour of water and other substances. What is this behaviour of water called?
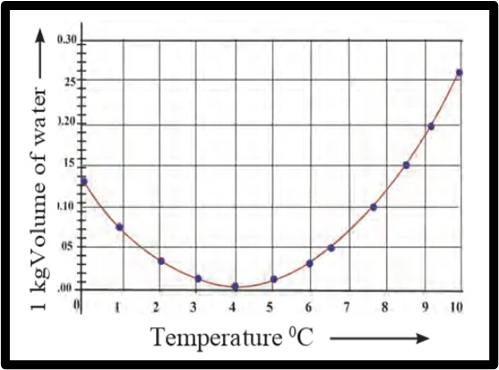
Answer: When the temperature of water increases from 0 °C to 10 °C, its volume decreases between 0 °C and 4 °C, reaching a minimum at 4 °C. However, the volume of water increases between 4 °C and 10 °C.
Typically, as a substance is heated, its volume increases with temperature. Therefore, the behaviour of water in the range of 0 °C to 4 °C differs from that of other substances, which is known as the anomalous behaviour of water.
Question 3. What is meant by specific heat capacity? How will you prove experimentally that different substances have different specific heat capacities?
Answer. The specific heat capacity of an object is the amount of heat energy needed to raise the temperature of a unit mass of the object by 1 °C.
Question 4. While deciding the unit for heat, which temperature interval is chosen? why?
Answer. When determining the unit for heat as a calorie, the chosen temperature interval is 14.5oC to 15.5oC. It is understood that the heat released or absorbed by a body can be calculated as ΔQ=msΔT. Additionally, one calorie is defined as the heat required to raise the temperature of 1 gram of water by 1oC. Therefore, for 1 calorie of heat energy, the specific heat capacity of water should be 1 calorie per gram per degree Celsius. Through experimental findings, it is established that the specific heat capacity of water is 1 calorie per gram per degree Celsius within the temperature range of 14.5oC to 15.5oC.
Question 5. Explain the following temperature vs time graph:
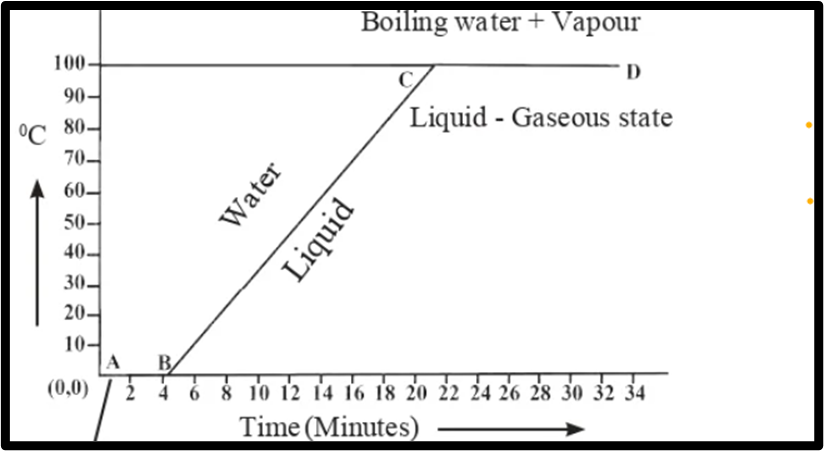
Answer. The graph illustrates the continuous heating of a mixture of ice and water. The temperature of the mixture stays constant at 0 °C until all the ice melts, marked by line AB, which indicates the melting point of ice. Subsequently, the temperature increases steadily from 0 °C to 100 °C, as depicted by line BC. At 100 °C, water transforms into steam, representing the boiling point of water. Continued heating maintains the temperature, and the conversion to steam persists, as shown by line CD.
Question 6. Explain the following:
a. the role of the anomalous behaviour of water in preserving aquatic life in regions of cold climate?
Answer. In cold regions during winter, the atmosphere temperature drops below 0 °C. This causes the water on lakes and ponds surfaces to contract, increasing its density and leading it to sink. This process continues until the water in the lake reaches a temperature of 4 °C. As the surface water cools further and falls below 4 °C, it begins to expand rather than contract, reducing its density and keeping it at the surface. The surface water temperature continues to drop to 0 °C. Eventually, the surface water freezes into ice, while the water below remains at 4 °C. Ice is a poor heat conductor, preventing heat transfer from the water to the atmosphere. This allows fish, aquatic animals, and plants to survive in the water below the ice layer.
b. How can you relate the formation of water droplets on the outer surface of a bottle taken out of a refrigerator with formation of dew?
Answer. At a specific temperature, air can only hold a certain amount of water vapor. As temperature decreases, the air’s ability to retain water vapor also decreases. When a bottle is placed in a refrigerator, its temperature drops below room temperature. Therefore, when the bottle is removed from the refrigerator, the surrounding air cools down, reducing its capacity to hold water vapor. Consequently, the excess water vapor condenses into water droplets on the bottle’s exterior, similar to dew formation.
c. In cold regions in winter, the rocks crack due to anomalous expansion of water.
Answer. Water can seep into rock crevices. If the temperature drops below 4 °C, the water will expand. When the water freezes and turns into ice, its volume increases. The lack of space for expansion causes it to exert immense pressure on the rocks, leading them to crack and break into smaller pieces.
Question 7.
a. What is meant by latent heat? How will the state of matter transform if latent heat is given off?
Answer. The latent heat of a substance is the quantity of heat needed to convert one unit of its mass from solid to liquid or from liquid to gas, with no change in temperature. When latent heat is released, the substance in liquid form will solidify and the substance in vapor form will turn into liquid. Consequently, the material’s internal energy decreases when latent heat is released.
b. Which principle is used to measure the specific heat capacity of a substance?
Answer. The principle of heat exchange is utilized to determine the specific heat capacity of a substance. In this scenario, when two objects are isolated within a heat-resistant box, no energy can exit or enter the box. The equation here is that the heat energy lost by the hot object equals the heat energy gained by the cold object
c. Explain the role of latent heat in the change of state of a substance.
Answer. When a solid is heated, its initial response is an increase in temperature. The heat absorbed by the substance is used to elevate the kinetic energy of its particles and to counteract the forces of attraction between them. As heating continues, the solid reaches a specific temperature known as the melting point, at which it transitions into a liquid state.
During this process, the temperature remains constant while the absorbed heat weakens the bonds within the substance, facilitating the shift to the liquid phase. This absorbed heat is termed the latent heat of fusion. Similarly, when a liquid transitions into a gaseous state at its boiling point, the absorbed heat is utilized to break the bonds between atoms or molecules, known as the latent heat of vaporization.
Some solids undergo direct transformation into a gaseous phase under specific conditions, where the absorbed heat is employed to break the bonds between atoms or molecules, leading to the phase change. This heat is referred to as the latent heat of sublimation. In essence, latent heat is the heat either absorbed or released by a substance during a state change at a constant temperature. When transitioning from liquid to solid, gas to liquid, and gas to solid, the substance emits latent heat.
d. what basis and how will you determine whether air is saturated with vapour or not?
Answer. The presence of water vapor in the air is determined by the amount of water vapor it contains. When the relative humidity reaches 100%, the air is fully saturated with water vapor, leading to the formation of water droplets on plant leaves or grass. If the relative humidity is less than 100%, the air is not saturated with water vapor.
Question 8. Read the following paragraph and answer the questions:
If heat is exchanged between a hot and cold object, the temperature of the cold object goes on increasing due to gain of energy and the temperature of the hot object goes on decreasing due to loss of energy.
Answer. The temperature change continues until both objects reaches the same temperature. During this process, the cold object absorbs heat energy while the hot object releases heat energy. When the system is isolated within a heat-resistant box, energy exchange only occurs between the two objects, preventing energy from entering or exiting the box.
(1) Heat is transferred from a hot object to a cold object. (2) This process illustrates the principle of heat transfer. (3) The cold object gains heat energy while the hot object loses energy. When a system of two objects is isolated, the heat energy lost by the hot object equals the heat energy gained by the cold object.
(4) This principle is utilized to determine the specific heat capacity of a substance
Question 9.
Solve the following problems:
a. Equal heat is given to two objects A and B of mass 1 g. The temperature of A increases by 3 °C and B by 5°C. Which object has more specific heat? And by what factor?
Solution. Data: m = 1 g, Δ T1 = 3 °C, Δ T2 = 5 °C,
Q same
Here, Q = mc1 ΔT1 = mc2 ΔT2
Thus, c1 > c2
The specific heat of A is more than that of B and
The specific heat of A = 5
The specific heat of B 3
b. Liquid ammonia is used in ice factory for making ice from water. If water at 20 °C is to be converted into 2 kg, ice at 0 °C, how many grams of ammonia is to be evaporated?
(Given: The latent heat of vaporization of 1 ammonia = 341 cal/g)
Solution:
Data: m1 = 2kg, ΔT1=20 °C – 0 °C
= 20 °C, c1 = 1 kcal/kg·°C, L1 (ice) = 80 kcal/kg,
L2 (vaporization of ammonia) = 341 cal/g = 341 kcal/kg, m2 =?
Q1 (heat lost by water) = m1c1 ΔT1 + m1L1
= 2kg × 1 kcal/kg·°C × 20 °C + 2 kg × 80 kcal/kg
=40 kcal + 160 kcal = 200 kcal
Q2 (heat absorbed by ammonia) = m2L2
= m2 × 34l kcal/kg
According to the principle of heat exchange, Q1 = Q2
∴ 200 kcal = m2 × 341 kcal/kg
∴ m2 = 200341 kg = 0.5864 kg = 586.4 g
586.4 g of ammonia are to be evaporated.
c. A thermally insulated pot has 150 g ice at temperature 0 °C. How much steam of 100 °C has to be mixed to it, so that water of temperature 50 °C will be obtained?
Solution:
(Given: Latent heat of melting of ice = 80 cal/g, latent heat of vaporization of water = 540 cal/g, specific heat of water = 1 cal/g °C)
Data: m1 = 150 g, ΔT1 = 50 °C – 0 °C
= 50 °C, cw = 1 cal/g.°C, L1 = 80 cal/g, L2 = 540 cal/g,
Δ T2 = 100°C – 50 °C = 50 °C, m2 = ?
Q1 (heat absorbed by ice) = m1L1
= 150 g × 80 cal/g = 12000 cal
Q2 (heat absorbed by water formed on melting of ice) =m1 cw ΔT1
= 150 g × 1 cal/g·°C × 50 °C = 7500 cal
Q3 (heat given out by steam) = m2L2
= m2 × 540 cal/g
Q4 (heat given out by water formed on condensation of steam)
= m2 cw ΔT2 = m2 × 1 cal/g·°C × 50 °C
According to the principle of heat exchange,
Q1 + Q2 = Q3 + Q4
∴ 12000 cal + 7500 cal = m2 × 540 cal/g + m2 × 50 cal/g
∴ 19500 cal = m2 (540 + 50) cal/g
∴ m2 = 19500590 g
33.5 g of steam is to be mixed.
d. A calorimeter has mass 100 g and specific heat 0.1 kcal/kg ·°C. It contains 250 g of liquid at 30 °C having specific heat of 0.4 kcal/kg·°C. If we drop a piece of ice of mass 10 g at 0 °C into the liquid, what will be the temperature of the mixture?
Solution:
Data: m1 = 100 g, c1 = 0.1 kcal/kg·°C,
= 0.1 cal/g·°C, T1 = 30 °C, m2 = 250 g,
c2 = 0.4 kcal/kg·°C = 0.4 cal/g·°C, T2 = 30 °C,
m3 = 10 g, T3 = 0 °C, L = 80 cal/g,
c (water) = 1 cal/g·°C, T = ?
Q1 (heat lost by calorimeter) = m1c1 (T- T1),
Q2 (heat lost by liquid) = m2c2 (T – T2),
Q3 (heat absorbed by ice) = m3 L,
Q4 (heat absorbed by water formed on melting of ice) = m3c (T – 0 °C)
According to the principle of heat exchange,
Q1 + Q2 = Q3 + Q4
∴ m1c1 (T1 – T) + m2c2 (T2 – T) = m3L + m3c (T – 0 °C)
∴ m1c1T1 – m1c1T + m2c2T2 – m2c2T = m3L + m3c (T – 0°C)
∴ m1c1T1 + m2c2T2 = m3L + (m1c1 + m2c2 + m3c)T
∴ 100g × 0.1 cal/g°C × 30 °C + 250g × 0.4 cal/g.°C × 30 °C J
= 10 g x× 80 cal/g + (100 g × 0.1 cal/g.°C + 250 g × 0.4 cal/g.°C + 10 g × 1 cal/g.°C) T
∴ (10 + 100 + 10) T = (300 + 3000 – 800)°C
∴ 120 T = 2500 °C
∴ T = 2500120 °C = 1256 °C = 20.83 °C
This is the temperature of the mixture.