Question 1: Identify the odd one out and justify.
(a) Chloride, nitrate, hydride, ammonium
- Odd one out: Ammonium
- Justification: Chloride (Cl⁻), nitrate (NO₃⁻), and hydride (H⁻) are all negatively charged ions (anions), while ammonium (NH₄⁺) is a positively charged ion (cation).
(b) Hydrogen chloride, sodium hydroxide, calcium oxide, ammonia
- Odd one out: Hydrogen chloride
- Justification: Sodium hydroxide (NaOH), calcium oxide (CaO), and ammonia (NH₃) are bases, while hydrogen chloride (HCl) is an acid.
(c) Acetic acid, carbonic acid, hydrochloric acid, nitric acid
- Odd one out: Acetic acid
- Justification: Acetic acid (CH₃COOH) is a weak organic acid, while carbonic acid (H₂CO₃), hydrochloric acid (HCl), and nitric acid (HNO₃) are strong inorganic acids.
(d) Ammonium chloride, sodium chloride, potassium nitrate, sodium sulphate
- Odd one out: Ammonium chloride
- Justification: Ammonium chloride (NH₄Cl) is a salt of a weak base (NH₄OH) and strong acid (HCl), which undergoes hydrolysis and shows acidic nature. The others (NaCl, KNO₃, Na₂SO₄) are neutral salts.
(e) Sodium nitrate, sodium carbonate, sodium sulphate, sodium chloride
- Odd one out: Sodium carbonate
- Justification: Sodium carbonate (Na₂CO₃) is a basic salt (forms basic solution in water due to hydrolysis), while sodium nitrate (NaNO₃), sodium sulphate (Na₂SO₄), and sodium chloride (NaCl) are neutral salts.
(f) Calcium oxide, magnesium oxide, zinc oxide, sodium oxide
- Odd one out: Zinc oxide
- Justification: Calcium oxide (CaO), magnesium oxide (MgO), and sodium oxide (Na₂O) are basic oxides, while zinc oxide (ZnO) is an amphoteric oxide (can act as both acid and base).
(g) Crystalline blue vitriol, crystalline common salt, crystalline ferrous sulphate, crystalline sodium carbonate
- Odd one out: Crystalline common salt
- Justification: Blue vitriol (CuSO₄·5H₂O), ferrous sulphate (FeSO₄·7H₂O), and sodium carbonate (Na₂CO₃·10H₂O) are hydrated salts that lose water of crystallization on heating, while common salt (NaCl) does not contain water of crystallization.
(h) Sodium chloride, potassium hydroxide, acetic acid, sodium acetate
- Odd one out: Acetic acid
- Justification: Sodium chloride (NaCl), potassium hydroxide (KOH), and sodium acetate (CH₃COONa) are electrolytes that dissociate completely in water, while acetic acid (CH₃COOH) is a weak electrolyte that dissociates partially.
Question 2: Write down the changes that will be seen in each instance and explain the reason behind it.
(a) 50ml water is added to 50ml solution of copper sulphate.
- Change: The blue color of the copper sulphate solution becomes lighter.
- Reason: Dilution reduces the concentration of Cu²⁺ ions, which are responsible for the blue color.
(b) Two drops of the indicator phenolphthalein were added to 10ml solution of sodium hydroxide.
- Change: The solution turns pink.
- Reason: Phenolphthalein is an indicator that turns pink in basic solutions. Sodium hydroxide (NaOH) is a strong base.
(c) Two or three filings of copper were added to 10ml dilute nitric acid and stirred.
- Change: Brown fumes of nitrogen dioxide (NO₂) are evolved, and the solution turns blue.
- Reason: Copper reacts with dilute nitric acid to form copper nitrate (blue solution) and nitrogen dioxide gas:
3Cu+8HNO3→3Cu(NO3)2+2NO+4H2O3Cu+8HNO3→3Cu(NO3)2+2NO+4H2O (NO oxidizes to NO₂ in air).
(d) A litmus paper was dropped into 2ml dilute HCl. Then 2ml concentrated NaOH was added to it and stirred.
- Change: Initially, blue litmus turns red (acidic). After adding NaOH, the solution becomes neutral or basic, so red litmus may turn blue.
- Reason: HCl is acid, NaOH is base. Neutralization occurs: HCl+NaOH→NaCl+H2OHCl+NaOH→NaCl+H2O.
(e) Magnesium oxide was added to dilute HCl and magnesium oxide was added to dilute NaOH.
- Change with HCl: Magnesium oxide dissolves, forming a colorless solution (magnesium chloride).
- Reason: MgO+2HCl→MgCl2+H2OMgO+2HCl→MgCl2+H2O (basic oxide reacts with acid).
- Change with NaOH: No reaction.
- Reason: Magnesium oxide is a basic oxide and does not react with bases.
(f) Zinc oxide was added to dilute HCl and zinc oxide was added to dilute NaOH.
- Change with HCl: Zinc oxide dissolves, forming zinc chloride solution.
- Reason: ZnO+2HCl→ZnCl2+H2OZnO+2HCl→ZnCl2+H2O (amphoteric oxide reacts with acid).
- Change with NaOH: Zinc oxide dissolves, forming sodium zincate solution.
- Reason: ZnO+2NaOH→Na2ZnO2+H2OZnO+2NaOH→Na2ZnO2+H2O (amphoteric oxide reacts with base).
(g) Dilute HCl was added to limestone.
- Change: Effervescence occurs due to carbon dioxide gas.
- Reason: CaCO3+2HCl→CaCl2+H2O+CO2↑CaCO3+2HCl→CaCl2+H2O+CO2↑
(h) Pieces of blue vitriol were heated in a test tube. On cooling, water was added to it.
- Change on heating: Blue crystals turn white (anhydrous CuSO₄).
- Reason: Loss of water of crystallization: CuSO4⋅5H2O→CuSO4+5H2OCuSO4⋅5H2O→CuSO4+5H2O
- Change on adding water: White powder turns blue.
- Reason: Rehydration: CuSO4+5H2O→CuSO4⋅5H2OCuSO4+5H2O→CuSO4⋅5H2O
(i) Dilute H₂SO₄ was taken in an electrolytic cell and electric current was passed through it.
- Change: Bubbles of gases (hydrogen and oxygen) are evolved at the electrodes.
- Reason: Electrolysis of acidified water:
- At cathode: 2H++2e−→H2↑2H++2e−→H2↑
- At anode: 4OH−→2H2O+O2↑+4e−4OH−→2H2O+O2↑+4e−
Question 3: Classify the following oxides into three types and name the types.
Oxides: CaO, MgO, CO₂, SO₃, Na₂O, ZnO, Al₂O₃, Fe₂O₃
- Basic oxides: CaO (calcium oxide), MgO (magnesium oxide), Na₂O (sodium oxide), Fe₂O₃ (ferric oxide).
(They react with acids to form salt and water.) - Acidic oxides: CO₂ (carbon dioxide), SO₃ (sulphur trioxide).
(They react with bases to form salt and water.) - Amphoteric oxides: ZnO (zinc oxide), Al₂O₃ (aluminium oxide).
(They react with both acids and bases to form salt and water.)
Question 4: Explain by drawing a figure of the electronic configuration.
(a) Formation of sodium chloride from sodium and chlorine:
- Sodium (Na, atomic number 11) has electron configuration 2,8,1. It donates 1 electron to achieve stable octet, forming Na⁺.
- Chlorine (Cl, atomic number 17) has electron configuration 2,8,7. It accepts 1 electron to achieve stable octet, forming Cl⁻.
- The oppositely charged ions attract to form ionic compound NaCl.
- Electronic configuration:
- Na: 2,8,1 → Na⁺: 2,8
- Cl: 2,8,7 → Cl⁻: 2,8,8
(b) Formation of magnesium chloride from magnesium and chlorine:
- Magnesium (Mg, atomic number 12) has electron configuration 2,8,2. It donates 2 electrons to form Mg²⁺.
- Two chlorine atoms (each Cl: 2,8,7) each accept 1 electron to form two Cl⁻ ions.
- The ions combine to form MgCl₂.
- Electronic configuration:
- Mg: 2,8,2 → Mg²⁺: 2,8
- Cl: 2,8,7 → Cl⁻: 2,8,8
Question 5: Show the dissociation of the following compounds on dissolving in water, with the help of chemical equation and write whether the proportion of dissociation is small or large.

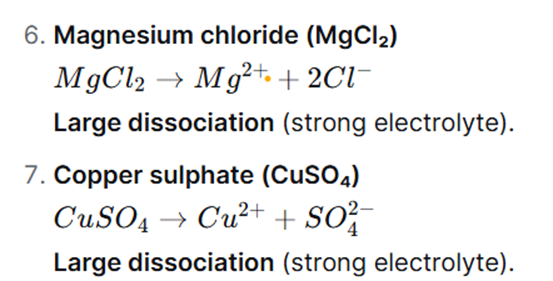
Question 6: Write down the concentration of each of the following solutions in g/L and mol/L.
General Formulas:
- Concentration in g/L = (mass of solute in grams / volume of solution in liters)
- Concentration in mol/L (Molarity) = (mass of solute in grams / molar mass) / volume of solution in liters
a. 7.3g HCl in 100ml solution
- Molar mass of HCl = 1 + 35.5 = 36.5 g/mol
- Volume = 100 mL = 0.1 L
- Concentration in g/L = 7.3g / 0.1L = 73 g/L
- Moles of HCl = 7.3 / 36.5 = 0.2 mol
- Concentration in mol/L = 0.2 mol / 0.1 L = 2 mol/L
b. 2g NaOH in 50ml solution
- Molar mass of NaOH = 23 + 16 + 1 = 40 g/mol
- Volume = 50 mL = 0.05 L
- Concentration in g/L = 2g / 0.05L = 40 g/L
- Moles of NaOH = 2 / 40 = 0.05 mol
- Concentration in mol/L = 0.05 mol / 0.05 L = 1 mol/L
c. 3g CH₃COOH in 100ml solution
- Molar mass of CH₃COOH = 12 + 3(1) + 12 + 16 + 16 + 1 = 60 g/mol
- Volume = 100 mL = 0.1 L
- Concentration in g/L = 3g / 0.1L = 30 g/L
- Moles of CH₃COOH = 3 / 60 = 0.05 mol
- Concentration in mol/L = 0.05 mol / 0.1 L = 0.5 mol/L
d. 4.9g H₂SO₄ in 200ml solution
- Molar mass of H₂SO₄ = 2(1) + 32 + 4(16) = 98 g/mol
- Volume = 200 mL = 0.2 L
- Concentration in g/L = 4.9g / 0.2L = 24.5 g/L
- Moles of H₂SO₄ = 4.9 / 98 = 0.05 mol
- Concentration in mol/L = 0.05 mol / 0.2 L = 0.25 mol/L
Question 7: Answer the following questions.
a. Classify the acids according to their basicity and give one example of each type.
- Basicity of an acid is the number of replaceable H⁺ ions per molecule.
- Monobasic acid: Has one replaceable H⁺ ion. Example: HCl (Hydrochloric acid)
- Dibasic acid: Has two replaceable H⁺ ions. Example: H₂SO₄ (Sulphuric acid)
- Tribasic acid: Has three replaceable H⁺ ions. Example: H₃PO₄ (Phosphoric acid)
b. What is meant by neutralization? Give two examples from everyday life of the neutralization reaction.
- Neutralization is a chemical reaction between an acid and a base to form salt and water.
- Examples:
- Antacid tablets: Used to neutralize excess stomach acid (HCl) with bases like magnesium hydroxide.
- Toothpaste: Neutralizes acid in the mouth (from bacteria) to prevent tooth decay.
c. Explain what is meant by electrolysis of water. Write the electrode reactions and explain them.
- Electrolysis of water is the process of decomposing water (H₂O) into hydrogen and oxygen gases by passing an electric current through it.
- Electrode reactions:
- At cathode (negative electrode): Reduction occurs.
2H2O+2e−→H2+2OH−2H2O+2e−→H2+2OH− (Hydrogen gas is evolved) - At anode (positive electrode): Oxidation occurs.
4OH−→O2+2H2O+4e−4OH−→O2+2H2O+4e− (Oxygen gas is evolved)
- At cathode (negative electrode): Reduction occurs.
- Overall reaction: 2H2O→2H2+O22H2O→2H2+O2
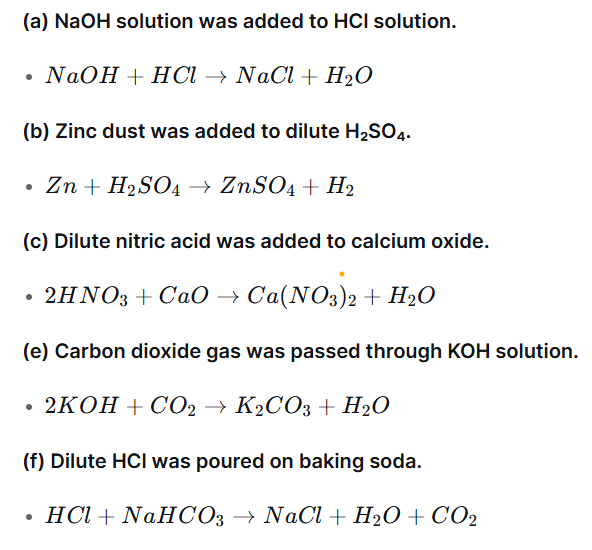
Question 8: Write the chemical equations for the following activities.
Question 9: State the differences.
a. Acids and bases
| Acids | Bases |
| Sour taste | Bitter taste |
| Turn blue litmus red | Turn red litmus blue |
| pH less than 7 | pH greater than 7 |
| Donate H⁺ ions | Accept H⁺ ions or donate OH⁻ ions |
b. Cation and anion
| Cation | Anion |
| Positively charged ion | Negatively charged ion |
| Formed by loss of electrons | Formed by gain of electrons |
| Attracted to cathode (negative electrode) | Attracted to anode (positive electrode) |
c. Negative electrode and positive electrode
| Negative electrode (Cathode) | Positive electrode (Anode) |
| Connected to negative terminal of battery | Connected to positive terminal of battery |
| Reduction occurs here | Oxidation occurs here |
| Cations are attracted to it | Anions are attracted to it |
Question 10: Classify aqueous solutions of the following substances according to their pH into three groups: 7, more than 7, less than 7.
Substances: Common salt, sodium acetate, hydrochloric acid, carbon dioxide, potassium bromide, calcium hydroxide, ammonium chloride, vinegar, sodium carbonate, ammonia, sulphur dioxide.
- pH = 7 (Neutral):
- Common salt (NaCl)
- Potassium bromide (KBr)
- pH > 7 (Basic):
- Sodium acetate (CH₃COONa)
- Calcium hydroxide (Ca(OH)₂)
- Sodium carbonate (Na₂CO₃)
- Ammonia (NH₃)
- pH < 7 (Acidic):
- Hydrochloric acid (HCl)
- Carbon dioxide (CO₂) [forms carbonic acid]
- Ammonium chloride (NH₄Cl)
- Vinegar (contains acetic acid)
- Sulphur dioxide (SO₂) [forms sulphurous acid]