Periodic Classification Element part 1 chapter2 Maharashtra board
Periodic Classification Element part 1 chapter2 Maharashtra board
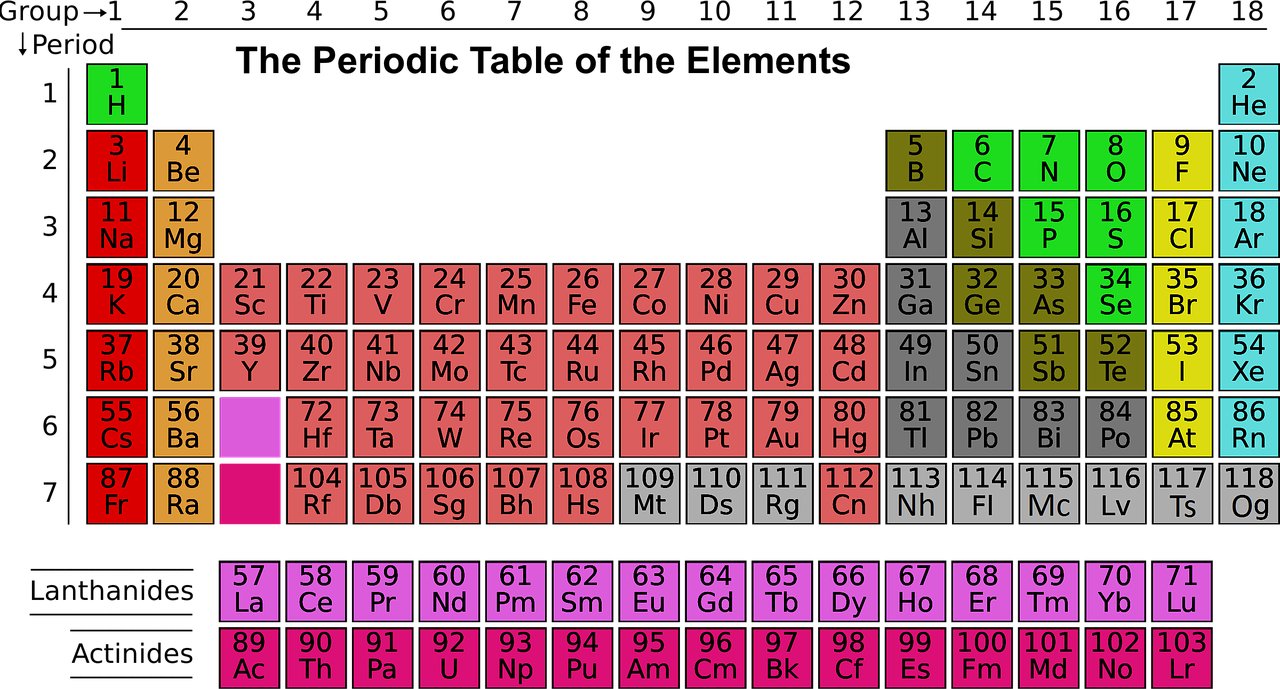
About Course
Periodic Classification of Elements Science 1 question1
| Column 1 | Column 2 | Column 3 |
| i. Triad | a.Lightest and negatively charged particle in all the atoms | 1.Mendeleev |
| ii. Octave | concentrated mass and positive charge | 2. Thomson |
| i. Atomic number | c. Average of the first and the third atomic mass | 3. Newlands |
| iv. Period | d. The properties of the eighth element are similar to the first 4 | 4. Rutherford |
| v. Nucleus | e. Positive charge on the nucleus | 5.Dobereiner |
| vi. Electron | f. Sequential change in molecular formulae | 6. Moseley |
Answer:
| Column 1 | Column 2 | Column 3 |
| i. Triad | Average of the first and the third atomic mass | Dobereiner |
| ii. Octave | Properties of the eighth element similar to the first | Newlands |
| iii. Atomic number | Positive charge on the nucleus | Moseley |
| iv. Period | Sequential change in molecular formulae | Mendeleev |
| v. Nucleus | Concentrated mass and positive charge | Rutherford |
| vi. Electron | Lightest and negatively charged particle in all the atoms | Thomson |
Periodic Classification Of Elements Science 1 Class 10 Maharashtra Board Question 2.
Choose the correct option and rewrite the statement:
(a) The number of electrons in the outermost shell of alkali metals is…….
(a) 1
(b) 2
(c) 3
(d) 7
Answer:
(a) 1
(b) Alkaline earth metals have valency 2. This means that their position in the modern periodic table is in…….
(a) Group
(b) Group 16
(c) Period 2
(d) d-block
Answer:
(a) Group 2
(c) The molecular formula of the chloride of an element X is XCl. This compound is a solid with having high melting point. which of the following elements are present in the same group as X?
(a) Na
(b) Mg
(c) Al
(d) Si
Answer:
(a) Na
(d) In which block of the modem periodic table are the nonmetals found?
(a) s-block
(b) p-block
(c) d-block
(d) f-block
Answer:
(b) p-block
Class 10 Science Chapter 2 Periodic Classification Of Elements Notes Question 3.
An element has its electron configuration as 2, 8, 2. Now answer the following questions.
a. What is the atomic number of this element?
Answer:
The atomic number of this element is 12.
- What is the group of this element?
Answer:
The group of this element is 2. - To which period does this element belong?
Answer:
This element belongs to period 3. - With which of the following elements would this element resemble? (Atomic numbers are given in the brackets)
N(7), Be(4), Ar(18), Cl(17)
Answer:
This element resembles Be(4).
Chapter 2 Class 10 Science 1 Periodic Classification Of Elements Question 4.
Write down the electronic configuration of the following elements from the given atomic numbers. Answer the following question with an explanation.
- 3Li, 14Si, 2He, 11Na, 15P which of these elements belong to be period 3?
Answer:
| Elements | Electronic configuration |
| (i) 3Li | 2,1 |
| (ii) 14Si | 2, 8,4 |
| (iii) 2He | 2 |
| (iv) 11Na | 2, 8, 1 |
| (v) 15P | 2, 8, 5 |
Elements belong to the 3rd period: 14Si, 11Na and 15P.
- 1H, 7N, 20Ca, 16S, 4Be, 18Ar. Which of these elements belongs to the second group?
Answer:
| Elements | Electronic configuration |
| (i) 1H | 1 |
| (ii) 7N | 2, 5 |
| (iii) 20Ca | 2, 8, 8, 2 |
| (iv) 16S | 2, 8, 6 |
| (v) 4Be | 2, 2 |
| (iv) 18Ar | 2, 8, 8 |
Elements belong to the 2nd group: 4Be and 20Ca.
- 7N, 6C, 8O, 5B, 13Al Which is the most electronegative element among these?
Answer:
| Elements | Electronic configuration |
| (i) 7N | 2,5 |
| (ii) 6C | 2,4 |
| (iii) 80 | 2,6 |
| (iv) 5B | 2,3 |
| (v) 13A1 | 2, 8,3 |
Among these, 8O is the most electronegative element.
- 4Be, 6C, 8O, 5B, 13Al Which is the most electropositive element among these?
Answer:
| Elements | Electronic configuration |
| (i) 4Be | 2, 2 |
| (ii) 6C | 2, 4 |
| (iii) 8O | 2, 6 |
| (iv) B | 2, 3 |
| (v) 11Al | 2, 8, 3 |
Among these, 13Al is the most electropositive element.
- 11Na, 15P, 17Cl, 14Si, 12Mg which of these has largest atoms?
Answer:
| Elements | Electronic configuration |
| (i) 11Na | 2, 8, 1 |
| (ii) 15P | 2, 8, 3 |
| (iii) 17Cl | 2, 8, 7 |
| (iv) 14Si | 2, 8, 4 |
| (v) 12Mg | 2, 8, 2 |
11Na has the largest atomic size.
- 19K, 3li, 11Na, 4Be Which of these atoms has smallest atomic radius?
Answer:
| Elements | Electronic configuration |
| (i) 19K | 2, 8, 8, 1 |
| (ii) 3Li | 2, 1 |
| (iii) 11Na | 2, 8, 1 |
| (iv) 4Be | 2, 2 |
4Be has the smallest atomic radius.
- 13Al, 14Si, 11Na, 12Mg, 16S Which of the above elements has the highest metallic character?
Answer:
| Elements | Electronic configuration |
| (i) 13Al | 2, 8, 3 |
| (ii) 14Si | 2, 8, 4 |
| (iii) 11Na | 2, 8, 1 |
| (iv) 12Mg | 2, 8, 2 |
| (v) 16S | 2, 8, 6 |
11Na has the highest metallic character.
- 6C, 3Li, 9F, 7N, 8O Which of the above elements has the highest nonmetallic character?
Answer:
| Elements | Electronic configuration |
| (i) 6C | 2, 4 |
| (ii) 3Li | 2, 1 |
| (iii) 9F | 2, 7 |
| (iv) 7N | 2, 5 |
| (v) 8O | 2, 6 |
9F has the highest nonmetallic character.
Chapter 2 Science 1 Class 10 Maharashtra Board Question 5.
Write the name and symbol of the element from the description.
a. The atom having the smallest size.
Answer: The atom having the smallest size is Helium (He)
- The atom having the smallest atomic mass.
Answer: The atom having the smallest atomic mass is Hydrogen (H2) - The most electronegative atom.
Answer: The most electronegative atom is Fluorine(F2). - The noble gas with the smallest atomic radius.
Answer: The noble gas with the smallest atomic radius is Helium (He). - The most reactive nonmetal.
Answer: The most reactive nonmetal is Fluorine(F2).
Periodic Classification of Elements Class 10 Science Chapter 2 Question 6.
Write short notes.
- Mendeleev’s periodic law.
Answer: Mendeleev’s periodic law states that the properties of the elements are periodic functions of their atomic masses. This means that elements with similar properties tend to have similar atomic masses. Mendeleev arranged the elements in his periodic table in order of increasing atomic mass, and he found that the elements with similar properties fell into vertical columns, called groups.
- Structure of the modern periodic table
The modern periodic table is based on the atomic number of the elements, not their atomic mass. The atomic number is the number of protons in the nucleus of an atom. The modern periodic table is arranged in 18 groups and 7 periods. The groups are vertical columns, and the periods are horizontal rows.
- Position of isotopes in Mendeleev’s and the modern periodic table
Isotopes are atoms of the same element with different atomic masses. They have the same number of protons, but different numbers of neutrons. Mendeleev’s periodic table was not able to accommodate isotopes because it was arranged by atomic mass. The modern periodic table is arranged by atomic number, so isotopes of the same element are placed in the same row.
Periodic Classification of Elements Class 10 State Board Question 7.
Write scientific reasons.
a. Atomic radius goes on decreasing while going from left to right in a period.
Answer. This is because the number of protons in the nucleus increases as you move from left to right in a period, while the number of electrons remains the same. This increase in nuclear charge pulls the electrons closer to the nucleus, resulting in a smaller atomic radius.
- The metallic character decreases while going from left to right in a period.
Metallic character is the tendency of an element to lose electrons and form positive ions. As you move from left to right in a period, the nuclear charge increases, making it more difficult for the atom to lose electrons. Therefore, metallic character decreases from left to right in a period.
The atomic radius goes on increasing down a group.
This is because the number of electron shells increases as you go down a group. Each electron shell is further away from the nucleus than the one before it, so the electrons are less strongly attracted to the nucleus and the atomic radius increases.
- Elements belonging to the same group have the same valency.
Valency is the number of electrons that an atom can lose, gain, or share to form a chemical bond. The valency of an element is determined by the number of electrons in the outermost electron shell. Elements in the same group have the same number of electrons in their outermost electron shell, so they have the same valency. - The third period contains only eight elements even though the electron capacity of the third shell is 18.
Answer. This is because the third shell can only hold up to 18 electrons, but only eight of these electrons can be valence electrons. The other 10 electrons must be in inner electron shells. The third period only contains elements with up to eight valence electrons, so it only contains eight elements.
here is the explanation for why the third period of the periodic table contains only 8 elements, even though the electron capacity of the third shell is 18:
The third period of the periodic table corresponds to the filling of the 3s and 3p subshells. The 3s subshell can hold up to 2 electrons, and the 3p subshell can hold up to 6 electrons. So, in total, the third period can hold up to 2 + 6 = 8 electrons.
However, not all of the elements in the third period have 8 electrons. The first two elements in the third period, sodium and magnesium, only have 1 and 2 valence electrons, respectively. This is because they have only filled the 3s subshell. The remaining 6 elements in the third period, aluminum, silicon, phosphorus, sulfur, chlorine, and argon, have 3 valence electrons. This is because they have filled both the 3s and 3p subshells.
So, even though the third shell can hold up to 18 electrons, only 8 of these electrons can be valence electrons. Therefore, the third period of the periodic table can only contain 8 elements.
Exercise Periodic Classification Of Elements Question 8.
Write the names from the description.
a. The period with electrons in the shells, K, L, and M.
Answer: The period with electrons in the shells, K, L, and M are third-period
- The group with valency zero.
Answer: The group with valency zero is group 18. - The family of nonmetals having valency one.
Answer: The family of nonmetals having valency one is the Halogen family - The family of metals having valency one.
Answer: The family of metals having valency one is Group 1. - The family of metals having valency two.
Answer: The family of metals having valency two in Group 2 - The metalloids in the second and third periods.
Answer: The metalloids in the second and third periods are Boron and silicon. - Nonmetals in the third period.
Answer: Nonmetals in the third period are phosphorus, sulfur chlorine, and argon. - Two elements having valency 4.
Answer: Two elements having valency 4 are Carbon and silicon.